Genomics: Insight
Wnt4 in the Development of Endometriosis
Research Question: With focus on Wnt4 and its effect in the development of endometriosis, what future steps can researchers take to address research gaps?
Over the years, healthcare has been diversifying to combat facets of the human population, especially lesser-known conditions and those affecting minorities. Endometriosis is one of these lesser researched disorders - but the cause isn’t because it’s rare. It’s a chronic condition affecting 10% of biological females of reproductive age in the world (190 million), and 1 in 10 in the US, making it much more common than one may think11.
In this condition, tissue similar to the inner lining of the uterus grows outside, forming inflammatory cysts, scar tissue, and clusters of fibrous tissue, causing organs and pelvic tissue to stick together. This condition can even lead to infertility and endometrial cancer11. Unfortunately, there isn’t enough awareness nor research done on this disease, attributed to a variety of factors such as a lack of awareness existing even in the medical field, with doctors brushing off women's pain and telling them that it is only pain caused by their menstrual cycles. In the realm of research as well, endometriosis has, until recently, slowly been garnering research due to how complex it is - it’s a polygenic disease, or a disease caused by both environmental and multiple genetic factors. Additionally, even rarer diseases like Crohn’s disease receive 65 times more funding per patient than endometriosis9. This lack of research does cause deeper issues, such as how 75.2% of patients reported being misdiagnosed with another physical health or mental health1 rather than endometriosis.
The exploration of the gene Wnt4 in particular may serve as some sustenance. In the year of 2023, researchers at Oxford published the largest study to date of the genetics of endometriosis, and they discovered 42 areas across the genome that are associated to or increase the risk of endometriosis, identifying a range of genes including Wnt42. Wnt4 is part of the Wnt genes in the first chromosome, and provides instructions for producing a protein crucial for the formation of the female reproductive system through regulating Mullerian ducts, or structures developing into uterus, fallopian tubes, the cervix, etc12. This biological function in relation to endometriosis can be seen when knockout of Wnt4 elicited female masculinization in mice, and conditional ablation(a procedure allowing for targeted removal of specific cells) caused subfertility and other effects6 with Wnt4 being crucial in Mullerian duct formation. Wnt4 mutations and defects have also caused ovarian dysfunction and misshapen Mullerian ducts, symptoms similar to endometriosis and other reproductive disorders. The Wnt4 pathway also contributes in mediating cell differentiation such as osteogenic differentiation(bone tissue), myofibroblast differentiation(cells helping in tissue repair), dendritic cell differentiation(immune cells), etc14. In this same signaling pathway, Wnt4 has links to other similar conditions as endometriosis such as the promotion of colorectal cancer through Wnt4 overexpression and initiation of preeclampsia(a pregnancy complication) via Wnt4 under-expression14. Apart from the pathway, polymorphisms in the Wnt4 gene are considered as one of the most significant SNPs in endometriosis7, and Wnt4 is associated with endometriosis in varying ethnicities, hence why it is the center of this review.
With focus on Wnt4 and its effect in the development of endometriosis, what future steps can researchers take to address research gaps?
Endometriosis is known as the “silent” disease, but the people suffering shouldn’t have to be silent.
Research
SNPS
SNPs, or single nucleotide polymorphisms, are variations in human DNA where a single nucleotide is different from the original. SNPs in 1p36.12 region/locus alleles are associated with an increased risk of endometriosis10. There are numerous SNPs in this region, with links to gynecologic disorders.
Rs7521902 is one SNP, and through a study where 250 studies were meta-analyzed, this SNP was a highlighted association of endometriosis across Latin American, European, and Asian populations7. It’s additionally highlighted as a SNP associated with endometriosis, and found to have a susceptibility region for endometriosis10. The problem is that the functional significance of this is yet to be explained.
Another, Rs3820282, is indicated as a functional SNP linked to endometriosis, and it’s also linked to increased risk of any subtype of epithelial ovarian cancer, with a variant frequency of 20% in Caucasian people, and up to 55% in Asian populations10. This is a promising variant to study because for starters, it’s a pleiotropic variant, so it’s associated with multiple disease phenotypes. By studying the different diseases, their molecular mechanisms could weigh in on the mechanisms in endometriosis; for example, evaluating the molecular background in endometrial cancer can point to Wnt4’s involvement as a marker for estrogen activity, a hormone immensely involved in women’s reproductive health4. When looking at its molecular mechanism, rs3820282 upregulates the transcription of WNT4 which increases the uterine permissiveness to embryo invasion and the decreasing resistance to invasion by cancer8.
Protein
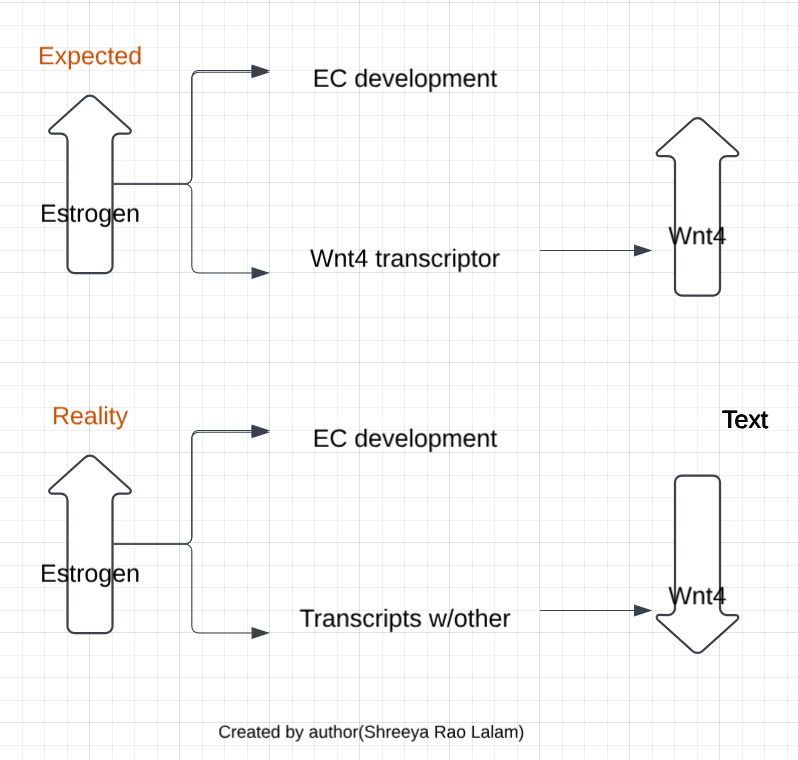
The protein coded for also is linked in the pathogenesis. In a study, the gene expression of Wnt4 was decreased in endometrial cancer samples compared to the control, but this was an unusual result because the predominance of estrogen activity triggers the development of EC, and estrogen acts as a transcription factor to Wnt4 so its expression should have increased4. However, estrogen can activate transcriptions of genes through interactions with other factors, so even though the tumor promoting effects of estrogen were kept, the expression of Wnt4 itself was decreased.
Research Gaps
Using the review conducted above, it’s clear there's been a lot of progress made so far, but there is still research needed in multiple sectors of endometriosis, both relating to the Wnt4 gene and in the broader range. Answers only come with more questions to be researched, deeper dives needed such as a further analysis of the genetic variants of Wnt4, especially when many of the studies related to their function came out recently. The lack of studies also poses another issue - variation in results. One study demonstrated that Wnt4 wasn’t associated with the menstrual cycle, but for another, the result was the opposite3.
In order to analyze Wnt4 in endometriosis more efficiently, models need to be used, but there’s little to no studies evaluating how different modeling approaches can impact the findings of pathology and immunology in endometriosis, and so further evaluation of animal models is needed to uncover more on the pathogenesis of endometriosis with the context of the research already conducted.
Another issue making endometriosis so difficult to catch or research is the absence of a nonsurgical biomarker for the diagnosis and surveillance of endometriosis3, hence why the condition is often conned “the silent disease”.
So, what can be done to address these gaps?
Steps Forward
The protein Wnt4 codes has a 98% similar amino acid identity to the Wnt4 protein of a mouse and rat, meaning animal models can be used in order to replicate endometriosis and examine its pathogenesis better13. In fact, there has been some information on mouse models, but it hasn’t been studied enough. For example, mice have many benefits to be used as animal models such as their smaller size, shorter estrous cycles, and it’s very common usage. However, mice don’t menstruate, and so an endometrium has to be injected or transplanted into them. The question of if it’ll be able to fit the lens of human endometriosis isn’t completely answered because it needs to resemble the immune response of the abdominal cavity, and the content of the implanted endometrium needs to be observed whether it influences the degree of the immune response3. By using an animal model, the elements found out during the research and results, such as the further influence of SNPs, can be studied. Finding a modeling approach can also spearhead the investigation of other components of endometriosis with relations to other genes.
Conclusion
Endometriosis is a complex disease, but with its impact on women and the lack of awareness that surrounds it societally, research needs to be done to support those who are suffering, and through investigating the genetics and mechanisms behind the disease, slowly but surely, the pieces may start to fall into place with better diagnostic approaches, biomarkers, more specific inklings into genes such as Wnt4 being found, etc. The most important thing is to support those affected by it however possible, and through identifying gaps in research and continuing to ask questions, more research is possible, as research runs on curiosity.
Endometriosis is known as the “silent” disease, but the people suffering don’t have to be.
- Charlton-Dailey, R. (2021, December 10). 90% of people with endometriosis report being dismissed by doctors and family. Verywell Health. https://www.verywellhealth.com/endometriosis-survey-disbelieved-5211872
- Global study shows the experience of Endometriosis is rooted in. (2023, March 14). https://www.ox.ac.uk/news/2023-03-14-global-study-shows-experience-endometriosis-rooted-genetics
- He, Y., Liang, B., Hung, S. W., Zhang, R., Xu, H., Chung, J. P. W., & Wang, C. C. (2022). Re-evaluation of mouse models of endometriosis for pathological and immunological research. Frontiers in Immunology, 13. https://doi.org/10.3389/fimmu.2022.986202
- Kiewisz, J., Waśniewski, T., Kieżun, J., Skowrońska, A., Kaczmarek, M. M., Szóstak, B., Kowalczyk, A. E., & Kmieć, Z. (2023). WNT4 Gene and protein Expression in endometrial cancer and its significance. Cancers, 15(19), 4780. https://doi.org/10.3390/cancers15194780
- Laganà, A. S., Garzon, S., Götte, M., Viganò, P., Franchi, M., Ghezzi, F., & Martin, D. C. (2019). The Pathogenesis of Endometriosis: Molecular and cell biology insights. International Journal of Molecular Sciences, 20(22), 5615. https://doi.org/10.3390/ijms20225615
- Mafra, F., Catto, M., Bianco, B., Barbosa, C. P., & Christofolini, D. (2015). Association of WNT4 polymorphisms with endometriosis in infertile patients. Journal of Assisted Reproduction and Genetics, 32(9), 1359–1364. https://doi.org/10.1007/s10815-015-0523-1
- Meidyana, S., Isfandiary, S., & Primariawan, R. Y. (2024). WNT4 (rs7521902 and rs16826658) polymorphism and its association with endometriosis – A systematic review and meta-analysis. European Journal of Obstetrics & Gynecology and Reproductive Biology, 295, 111–117. https://doi.org/10.1016/j.ejogrb.2024.01.038
- Pavličev, M., McDonough-Goldstein, C. E., Zupan, A. M., Muglia, L., Hu, Y., Kong, F., Monangi, N., Dagdas, G., Zupančič, N., Maziarz, J., Sinner, D., Zhang, G., Wagner, G., & Muglia, L. (2024). A common allele increases endometrial Wnt4 expression, with antagonistic implications for pregnancy, reproductive cancers, and endometriosis. Nature Communications, 15(1). https://doi.org/10.1038/s41467-024-45338-4
- Peter, R. M. (2023, October 17). Five latest advancements in endometriosis research. Labiotech.eu. https://www.labiotech.eu/best-biotech/endometriosis-research-latest-advancements/
- Pitzer, L. M., Moroney, M. R., Nokoff, N. J., & Sikora, M. J. (2021). WNT4 Balances Development vs Disease in Gynecologic Tissues and Women’s Health. Endocrinology, 162(7). https://doi.org/10.1210/endocr/bqab093
- World Health Organization: WHO & World Health Organization: WHO. (2023, March 24). Endometriosis. https://www.who.int/news-room/fact-sheets/detail/endometriosis
- WNT4 gene: MedlinePlus Genetics. (n.d.). https://medlineplus.gov/genetics/gene/wnt4/
- WNT4 Wnt family member 4 [Homo sapiens (human)] - Gene - NCBI. (n.d.). https://www.ncbi.nlm.nih.gov/gene/54361
- Zhang, Q., Pan, Y., Ji, J., Xu, Y., Zhang, Q., & Qin, L. (2021). Roles and action mechanisms of WNT4 in cell differentiation and human diseases: a review. Cell Death Discovery, 7(1). https://doi.org/10.1038/s41420-021-00668-w
About the Author
Shreeya Rao Lalam is a sophomore at Dublin High School in Dublin, California. She has an invested interest in both genetics and reproductive health, and aspires to delve deeper into medicine to hope to help those with conditions like endometriosis.
Mentor: Michael Correia Affiliation: Dublin High School