Genomics: Insight
The Investigation of Pharmacogenomics on Adverse Effects
Introduction
Pharmacogenomics is the study of how a person's genes affect their response to medicinal drugs. Experts use pharmacogenomics to create safe and effective medications and doses customized for a person's genetic makeup. This field of research is a part of precision medicine with the long-term goal of treating patients individually based on their genes. Most drugs that are available are made for everyone to use, but in many cases these drugs do not fit every person's needs because they can have severe adverse effects, also known as side effects. To make therapeutics more accessible to a larger population, one area that should be explored is controlling the adverse effects of drugs used for mental disorders, such as antidepressants, by genotyping and identifying genetic variants. This will allow for researchers to determine those genetic variants associated with adverse side effects and those variants that have no impact.
This field of research is a part of precision medicine with the long-term goal of treating patients individually based on their genes.
Genotyping is the process of determining the different single nucleotide polymorphisms (SNPs) by examining a person’s DNA sequence. SNPs are misspellings in a DNA sequence of the nucleotides adenine, guanine, cytosine, and thymine. SNPs can act as biological markers, which are an indication of a particular disease or adverse effect. If genotyping is used as a screening protocol, doctors could prescribe medications to people who do not have those specific variants associated with adverse effects.
Miller et. al (2019) states that two genes, Cytochrome P450 2D6 (CYP2D6) and Cytochrome P450 2C19 (CYP2C19), could be targeted in clinical trials to reduce severe adverse effects of drugs used to treat mental disorders. The drugs are tricyclic antidepressants (TCAs) and have a structure that contains tertiary and secondary amines. CYP2D6 metabolizes both the tertiary and secondary amines while CYP2C19 only metabolizes the tertiary amines (see Figure 1). TCAs are a type of antidepressant called serotonin reuptake inhibitors (SSRIs) and they inhibit the CYP2D6 and CYP2C19 enzymes, therefore increasing adverse side effects. Inhibiting the CYP2D6 and CYP2C19 gene can affect the enzyme's ability to metabolize drugs. If the body has poor metabolism then the adverse effects will become more severe.
If genotyping is used as a screening protocol, doctors could prescribe medications to people who do not have those specific variants associated with adverse effects.
Methods
A. Dosage Recommendations Based on Functional Metabolism Score
The functional metabolism score is one tool researchers have focused on to inform dosage recommendations of medicinal drugs. In one study, scientists collected DNA from patients and examined their genes through genotyping (Hicks et al., 2016). Researchers tested for SNPs in the CYP2D6 and CYP2C19 alleles. A scoring system was developed to determine the functional status of the alleles. For the CYP2D6 allele, a score of 2 or greater represented ultrarapid metabolization, a score of 1 represented normal function, a score of 0.5 represented decreased function, and a score of 0 represented no function. Patients with two normal CYP2C19 alleles were considered normal metabolizers. Patients with one or two nonfunctional CYP2C19 alleles were considered intermediate or poor metabolizers.
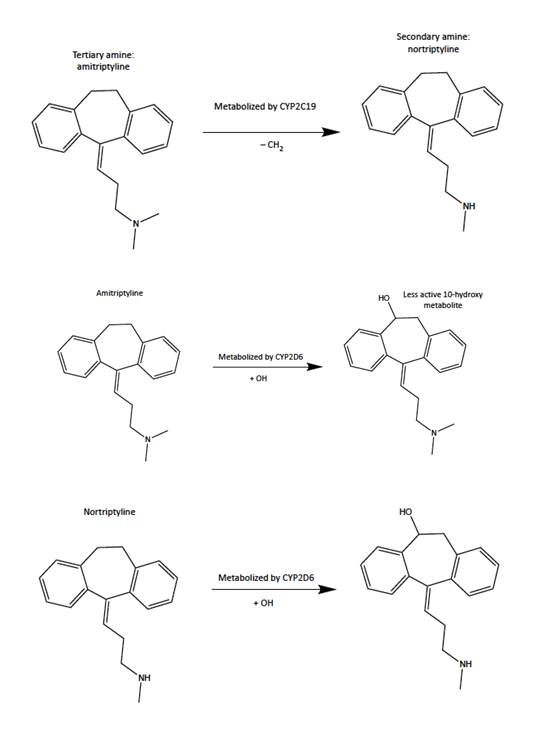 Figure 1: CYP2D6 and CYP2C19 are the enzymes in the cytochrome P450 gene family. CYP2C19 only metabolizes the amitriptyline (tertiary amine) which gets turned into the norttriptyline (secondary amine). CYP2D6 metabolizes amitriptyline which gets turned into a less active metabolite of nortriptyline. CYP2D6 then metabolizes nortriptyline.
Figure 1: CYP2D6 and CYP2C19 are the enzymes in the cytochrome P450 gene family. CYP2C19 only metabolizes the amitriptyline (tertiary amine) which gets turned into the norttriptyline (secondary amine). CYP2D6 metabolizes amitriptyline which gets turned into a less active metabolite of nortriptyline. CYP2D6 then metabolizes nortriptyline.B. Dosage Recommendations Based on NeuroIDgenetix Test Results
Another tool researchers have explored to inform dosage recommendations is the NeuroDgenetix Test. The purpose of this study was to evaluate the effectiveness of pharmacogenetic guided medicine. A study conducted across 20 clinical sites within the US randomized 685 patients by disease and disease severity, then assigned them to the experimental group (NeuroIDgenetix test) or control group (standard treatment). “The NeuroIDgenetix uses a genetic variant panel of ten genes along with concomitant medications, to make medication management recommendations based on gene-drug and drug-drug interactions for over 40 medications used in the treatment of depression and anxiety.” (Bradley et. al, 2017). Samples were collected from all patients using buccal swabs. Pharmacodynamic and pharmacokinetic variants associated with CYP2C19, CYP2D6 were tested. Results from the NeuroIDgenetix test of the experimental group were given to participating clinicians. The control group was also given the NeuroIDgenetix test, but their results were withheld. The experimental group’s results were given to clinicians two weeks in advance in order for them to make medication changes. Patients were assessed for symptoms of depression anxiety using the Hamilton Rating Scale for Anxiety (HAM-A) and Hamilton Rating Scale for Depression (HAM-D17). HAM-D17 and HAM-A are questionnaires used to recognize any indication of depression or anxiety in patients and is used to evaluate recovery. The assessments occurred at the start of the study and at 4th, 8th, and 12th week intervals. Adverse drug events were monitored by the clinicians as well as the patients.
Results
A. Dosage Recommendations Based on Functional Metabolism Score
Based on the genetic test results of CYP2D6 and CYP2C19, the researchers have created the Clinical Pharmacogenomics Implementation Consortium Guideline (CPIC). The guideline provides recommendations such as prescribing doses of TCAs based on the efficacy level of TCA metabolism which is associated with the CYP2D6 and CYP2C19 genes. The dosing efficacy levels are separated into the following groups: ultrarapid metabolizer, normal metabolizer, intermediate metabolizer, and poor metabolizer.
The recommendations include that CYP2D6 ultrarapid metabolizers should avoid using TCAs due to the rapid metabolization reducing the efficacy of the medication. If a TCA is needed, they should take larger doses than recommended for normal metabolizers. Normal metabolizers should start therapy with the recommended starting dosage since their metabolization allows the drug to be effective. Intermediate metabolizers should start with a 25% reduction of the recommended starting dosage. These patients have reduced metabolism which causes the TCAs to be more inactive; this would increase the probability that the patient will experience severe adverse effects. Poor metabolizers should avoid using TCAs and consider using a drug not dependent on the CYP2D6 metabolization, but if needed they should start with a 50% reduction of the recommended starting dosage. These patients have reduced metabolism that won’t completely break down the drug; this would increase the probability that the patient will experience severe adverse effects.
Recommendations for CYP2C19 ultrarapid and poor metabolizers include that they should avoid using TCAs. Instead, they should use drugs that aren’t metabolized by CYP2C19. If needed, patients should use therapeutic drug monitoring. Poor metabolizers should start with a 50% reduction of the recommended starting dosage. Normal and intermediate metabolizers should start therapy with the recommended starting dosage.
B. Dosage Recommendations Based on NeuroIDgenetix Test Results
Results from researchers Bradley et al. (2017) further supported the conclusions of the Hicks et al. (2016) study. The depression remission rate of the patients in the experimental group at the 8-week follow-up was 25%, while the depression remission rate of the control group was 9%. At the 12-week visit, the remission rate for the experimental group increased to 35% while the control groups increased to 13%. Response rates were also significantly higher for patients in the experimental group. At the 8-week follow-up the response rate for the experimental group compared to the control group was 55% versus 28% for patients diagnosed with severe depression. There was no significant change in patients diagnosed with mild depression. It was concluded that these patients may not benefit from TCAs.
For patients diagnosed with anxiety in the experimental group, their response rates were increased by 63%, while the control groups were increased by 50%. Based on the HAM-A scores, the clinicians noticed a reduction of anxiety symptoms for the patients in the experimental group. In order for the scores to be counted as significant patients had to obtain a score of 18 or less. HAM-A scores were reduced by 54%.
The NeuroIDgenetix test increased the depression and anxiety remission rates of the patients in the experimental group. Although the depression and anxiety remission rates of the patients in the control group also increased, it wasn’t as significant. This study showed that genotyping can be used as a screening tool to reduce the severity of the drug's side effects.
Conclusion
The use of genotyping as a screening tool can reduce the severe adverse effects of drugs such as antidepressants. The study done by Hicks et. al (2016) targeted the genes CYP2D6 and CYP2C19 of patients through genotyping. They also studied how the genes relate to the dosing of TCAs. Based on the metabolism of these two genes, a guideline recommending antidepressant dosages was created. The authors of this study have concluded that pharmacotherapy for patients with CYP2D6 and CYP2C19 could improve clinical outcomes and reduce the adverse effects.
The study done by Bradley et al. (2017) used the NeuroIDgenetix test to adjust the dosing of the experimental groups' medication based on their CYP2D6 and CYP2C19 gene variants. Following the medications suggested by the NeuroIDgenetix test, depression and anxiety remission rates increased significantly according to the HAM-D and HAM-A test. Results from the study further supported the conclusions of the Hicks et al. study.
These studies have shown that pharmacogenomics testing and genotyping can mitigate severe adverse effects. Future research could also involve the use of precision medicine to personalize medicine.
Future research could also involve the use of precision medicine to personalize medicine.
Related Links
- Bradley, P., Shiekh, M., Mehra, V., Vrbicky, K., Layle, S., Olson, M. C., … Lukowiak, A. A. (2017, September 23). Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: A randomized clinical trial demo... https://pubmed.ncbi.nlm.nih.gov/28992526/
- Hicks, J., Sangkuhl, K., Swen, J., Ellingrod, V., Müller, D., Shimoda, K., Bishop, J., Kharasch, E., Skaar, T., Gaedigk, A., Dunnenberger, H., Klein, T., Caudle, K. and Stingl, J. (2017), Clinical pharmacogenetics implementation consortium guideline... https://doi.org/10.1002/cpt.597
- Miller, John J. (2019, June 19). Psychiatric Pharmacogenomic Testing: The Evidence Base. Psychiatric Times. https://www.psychiatrictimes.com/view/psychiatric-pharmacogenomic-testing-evidence-base
- Psychiatric Pharmacogenomic Testing: The Evidence Base. Psychiatric Times. https://www.psychiatrictimes.com/view/psychiatric-pharmacogenomic-testing-evidence-base
- SD;, G. N. K. B. R. S. Human genome project: pharmacogenomics and drug development. Indian journal of experimental biology. https://pubmed.ncbi.nlm.nih.gov/11883519/
- U.S. Department of Health and Human Services. Pharmacogenomics. National Institute of General Medical Sciences. https://www.nigms.nih.gov/education/fact-sheets/Pages/pharmacogenomics.aspx
About the Author
Jasmine Bonds is currently a senior at Charles Herbert Flowers High School. She is interested in the topic pharmacogenomics and its role in producing precision medicine.