Genomics: Insight
Exploring the Gut-Brain Axis
Exploring the Gut-Brain Axis: How Do the Bacteria in our Gut Communicate with our Brain?
Hippocrates, over 2,000 years ago, attributed disease origins to the gastrointestinal system [1]. While these thoughts were undoubtedly ahead of their time, scientists now realize how accurate these statements may be. The gut microbiome, or the collection of microbes that inhabit our gastrointestinal tract, is thought to play an essential role in how human bodies function every day. While scientists are learning more about the associations of altered gastrointestinal microbes with health and disease, we are just starting to discover the mechanisms and pathways underlying these connections. With the emergence and improvement of genomic sequencing technology in recent years, the study of the microbiome has become more accessible and more affordable. This increased access has enabled the creation of large-scale scientific efforts to characterize which microbes inhabit which areas of the human body, an endeavor known as mapping the human microbiome, such as the Human Microbiome Project, funded by the National Institutes of Health [2, 3]. Given these advances in mapping the human microbiome, there is a growing interest in investigating how the microbiome is connected to the brain.
Overview of the Microbiome and the Gut-Brain Axis
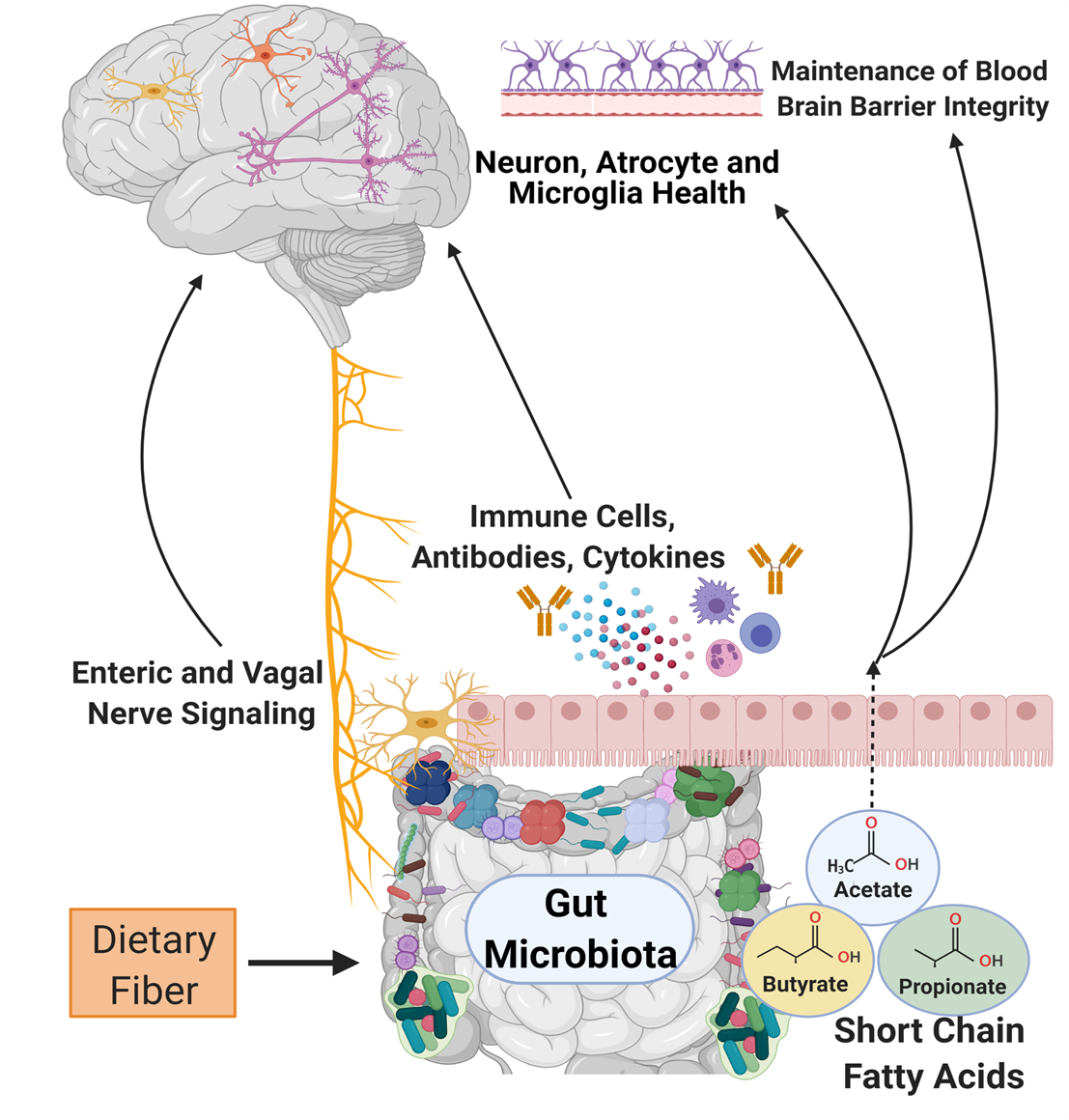
The bacteria of the human microbiota are commensal microorganisms, defined as organisms that live together and benefit from each other, that colonize multiple surfaces and niches of the body, including the gastrointestinal tract. These gastrointestinal bacteria perform vital physiological functions from nutrient metabolism to neurotransmitter release (>95% of the serotonin in the body, a critical hormone that stabilizes our mood, is produced in the gastrointestinal tract) [4]. Recently, scientists are publishing research that shows the influence of the gastrointestinal microbiota is not restricted to just the gastrointestinal tract; these bacteria also play a prominent role in the bidirectional communication between the gastrointestinal tract and the brain (entitled the gut-brain axis). Certain harmful changes in the gastrointestinal microbiome bacteria are associated with neurological and mental health disorders, including anxiety, autism, depression, Parkinson’s, and Alzheimer's disease [5]. These associations are exciting as they open up avenues to understand and treat brain disorders by studying and changing the gastrointestinal microbiota. Currently, we have a basic understanding of three routes through which the gut microbiota can communicate with the brain: metabolite signaling, the immune system, and/or the nervous system.
...the influence of the gut microbiota is not restricted to the gastrointestinal tract, but that these bacteria play a large role in bidirectional signaling between the gut and the brain...
Metabolite Signaling
As microbiome analysis technology advances, scientists can classify species of bacteria and understand what metabolic processes are increased or decreased when there are changes in the microbial environment. This data can help us understand the influence that microbiome alterations can have on the physiological processes outside of the gastrointestinal tract. Metabolites produced by the gut microbiota that have been the most heavily researched are short-chain fatty acids. Gut microbiota bacteria produce short-chain fatty acids (i.e., butyrate, acetate, and propionate) through the process of breaking down complex carbohydrates. They are involved with essential body functions in the intestine and between multiple body systems, including the brain. For example, short-chain fatty acids are involved in the maturation and development of host defender cells in the brain (microglia) through their regulation of free fatty acid receptor 2 (FFAR2) signaling [6]. Short-chain fatty acids are also associated with improved protection of the brain through the blood-brain-barrier. The blood-brain-barrier is the first line of defense against the invasion of large molecules and pathogens. If the body does not have enough short-chain fatty acids available, there is a breakdown of proteins that reinforce the blood-brain-barrier defense system [6].
Immune System
Immunoglobulin A (IgA) immune cells are designed to protect the body from viral and bacterial infections. As part of the gut-brain axis, IgA immune cells are trained by the gut microbiota bacteria to preserve the blood-brain-barrier. The IgA antibody-producing cells inhabit and defend the blood-brain-barrier surrounding the central nervous system to prevent the brain from being susceptible to infection. The notable importance of these microbially-trained IgA cells was demonstrated when removing IgA cells resulted in a higher incidence of fatal brain infections in mice [7]. In other words, mice given antibiotics that depleted these IgA cells in their body had a substantially decreased ability to fight infection. In humans, increased susceptibility to brain inflammation and infection can increase the risk for neurologic disorders such as Alzheimer’s disease and dementia [8]. IgA is not the only immune cell that plays a role in the gut-brain axis, but it is an apparent guardian against infection and downstream inflammation.
Nervous System
The enteric nervous system, the subset of the nervous system that controls parts of the gastrointestinal tract [9], and the vagus nerve are essential components of the bidirectional information transmission system of the gut-brain axis. The vagus nerve, also known as cranial nerve X and the main parasympathetic nerve of the body, helps relieve stress, originates in the brainstem, and extends to the gastrointestinal system. Gastrointestinal bacteria interact with and signal to the vagus nerve to facilitate communication and effects in the brain and spinal cord [10].
Specific bacterial strains, such as Campylobacter jejuni or Lactobacillus rhamnosus (JB-1), have been shown to activate or reduce expression in targeted brain regions (paraventricular hypothalamus, amygdala, and cingulate cortex, among others) in mice via communication with the vagus nerve [11,12]. Expression in these brain regions is vital for mood and nervous system regulation, and abnormal activation may be linked to increased risk for disorders ranging from depression to cardiovascular disease in humans [12, 13]. This is an open area of research to test and replicate the translational knowledge between animal models and humans.
The human enteric nervous system contains approximately 200-600 million neurons and has been described as the “second brain” within the body [14]. Nerves in the enteric nervous system directly interface with the bacteria of the gastrointestinal microbiota [15]. Bacterial metabolites like short-chain fatty acids can influence the enteric nervous system, further demonstrating the gut-brain axis is an interconnected but highly complex system [16].
Conclusion
Overall, researchers are still exploring the mechanisms through which the bacteria of the gastrointestinal microbiota may signal to the brain. Future research efforts focused on translational methodologies are opening new opportunities for insight. This information will increase understanding of how the microbiota can be modulated to influence, and possibly lower, disease susceptibility to neuropsychiatric disorders and improve human health.
Authors Note: The content is solely the authors' responsibility and does not necessarily represent the official views of the National Institutes of Health. Figures created with Biorender.
About the Author
Caitlin Dreisbach is a postdoctoral researcher at Columbia University. Her research uses data science techniques to uncover the biological and experiential complexities of maternal health during pregnancy.
Katherine and Caitlin are members of the International Society of Nurses in Genetics (ISONG) and are passionate about incorporating genomic methodologies and techniques into nursing research and education